In a world engrossed with uncertainty since the start of 2020, the COVID-19 pandemic has indisputably left a considerable impact on everyone’s lives. This unprecedented situation has challenged various sectors worldwide, particularly the public health system.
Understanding COVID-19 Test Kits
To effectively combat COVID-19 and protect public health, widespread testing capabilities are of utmost importance. Here is a comprehensive insight into COVID-19 test kits, their functionality, and their types.
Types of COVID-19 Test Kit
There are different types of COVID-19 test kits currently available
- PCR tests: Regarded as the pinnacle or the “gold standard” in the realm of COVID-19 testing, these kits have earned their remarkable reputation because of their cutting-edge ability to zero in on and identify the genetic material belonging to the virus. This, in essence, provides a highly detailed, accurate snapshot of whether the individual being tested has an active viral infection. In addition, the high sensitivity level of these testing kits significantly increases their reliability. The test is capable of picking up even tiny amounts of the virus, lowering the chances of false negatives, and ensuring that cases aren’t missed. This makes them a vital tool in our ongoing battle against the COVID-19 pandemic, helping accurately diagnose cases with high efficiency and playing a key role in global efforts to control the virus’s spread.
- Antigen tests: You might have often heard the term ‘rapid tests.’ These are a type of diagnostic tests that are gaining popularity because of their ability to provide fast results, usually within minutes. The way they function primarily involves the identification of proteins present on the surface of the virus that causes the disease. These proteins serve as telltale signs of viral presence and can help confirm if a person is currently infected. The beauty of these rapid tests lies in their speed because quick diagnosis spells early treatment and lessens the chances of the illness spreading to others. However, it’s essential to note that rapid tests are not without their limitations. One significant drawback is the higher chance of false-negative results compared to other testing methods. What that means is, that sometimes, the test might tell you that you’re not infected when in reality, you are. This could be due to various reasons like low viral load at the time of testing or the inability of the test to detect the virus accurately. In the end, while rapid tests offer a distinct advantage in terms of speed, they may not always be entirely reliable. Therefore, they should ideally be used in conjunction with other diagnostic methods to ensure accurate results.
- Antibody tests: These tests are designed to identify the existence of specific antibodies within the human body. Fundamentally, these antibodies are proteins that the immune system generates when it encounters harmful substances, such as bacteria or viruses. When they are present, it generally indicates that you had an infection in the past. However, it’s crucial to understand that these types of tests are not appropriate for detecting active or ongoing infections. The reason is, that it can take days to weeks for our bodies to produce antibodies after an infection sets in. Simply put, if you are currently infected and your body hasn’t yet generated antibodies, these tests may produce negative results. Using them to identify current infections could potentially lead to false negatives— an erroneous sense of security and potentially the further spread of the infection. Hence, while valuable for understanding past infections and possibly immunity, they are an unsuitable tool for detecting and managing active infections.
- Fluorescence Immunoassay Kits: Fluorescence immunoassay kits, wonderfully innovative tools in modern science, employ strategically used fluorescence markers in their mechanism. These markers can detect the presence of viral proteins or antibodies, providing vital clues regarding disease presence or progression. Enjoying widespread usage, these kits offer a robust and quantitative approach to measuring these components. In other words, these kits are not just able to detect a particular protein or antibody, they can also give a highly accurate measure of their concentration. This could prove pivotal when tracking the growth or decline of a particular virus or disease. Primarily utilized in laboratory settings, the use of fluorescence immunoassay kits is a part and parcel of many experimental procedures and studies. Scientists rely on them for providing detailed quantitative analysis, without which, it would be quite challenging to proceed with their research. This is a case of technology proving instrumental in aiding the discovery and understanding of intricate biological phenomena. Moreover, these kits display exceptionally high sensitivity which enhances their practical utility in these settings. This high sensitivity implies that even minute quantities of the target proteins or antibodies can be detected, thus making these kits extremely reliable in their results. This aspect can be crucial when dealing with issues where early detection is paramount. Although primarily used in research, these kits also find usage within clinical laboratories. Clinical labs often require accurate measurement of antibodies or proteins as part of medical diagnoses. In such scenarios, fluorescence immunoassay kits could offer a competent means of doing so. So, whether it’s for research or clinical purposes, fluorescence immunoassay kits truly bring a lot to the table. By allowing for highly accurate detection and detailed quantitative analyses, they undoubtedly play an essential role in modern laboratories.
- ELISA Tests (Enzyme-Linked Immunosorbent Assay): ELISA tests, short for Enzyme-Linked Immunosorbent Assay, utilize an enzyme to locate and identify viral antigens or antibodies from a sample taken from a patient. Such tests work by leveraging the enzyme’s ability to generate a detectable signal to highlight the presence of disease-specific proteins. These tests are a common tool deployed in laboratory environments, where they serve to facilitate in-depth analysis and bolster research efforts. Whether utilized to investigate a particular illness or to aid in the generation of comprehensive medical data, ELISA tests are quite integral in these settings due to their unique features and benefits. One of the standout attributes of ELISA tests is their high degree of sensitivity. This feature means that even a tiny amount of an antigen or antibody can trigger a positive signal, ensuring a remarkably precise detection. This ability to identify even traces of a substance or microorganism can greatly increase the chances of an early diagnosis and subsequent successful treatment, particularly in the case of infectious diseases or conditions. Another notable characteristic of ELISA tests is their extraordinary specificity. The specificity feature ensures the test’s ability to distinguish the target antigen or antibody from other molecules in the sample. This preciseness helps to significantly reduce the chances of false positive results and gives an accurate analysis. Lastly, ELISA tests offer quantitative results. This means that instead of simply indicating whether a sample is positive or negative, they provide detailed measurements of the amount of an antigen or antibody present. This capability makes ELISA tests an indispensable tool when it comes to tracking disease progression, monitoring treatment success, or studying biological processes in a research setting. With high sensitivity and specificity as well as their ability to provide detailed quantitative results, ELISA tests are a vital part of modern laboratory analysis and medical research.
- Rapid Tests (Lateral Flow Tests): Quick tests, which utilize the impressive power of lateral flow technology, serve as a convenient and rapid method of identifying viral antigens or antibodies present within the human body. This impressive and innovative technology is commonly used in immediate care scenarios, providing healthcare professionals with the essential insights to address a patient’s illness more effectively and promptly. In addition, these quick tests have found extensive applications in communal or public health testing efforts. By facilitating mass testing, they help identify and isolate cases quickly, enabling health authorities to manage and contain potential outbreaks more efficiently. They have proven to be great assets, particularly in crowded places, social events, and public gatherings where prompt identification and management of potential infections are critical. There’s also an increasing deployment of these tests in circumstances where quick and immediate results are profoundly needed. This could range anywhere from airport terminals for pre-flight and post-flight testing or border checks to corporate settings and educational institutions to carry out routine health screenings. While the rate of sensitivity – that is, the ability of tests to correctly identify those with the infection – may vary across different brands or types of quick tests, they continue to be well-appreciated by many. What most folks appreciate about these tests is their speedy delivery of results. Those nerve-wracking minutes of anticipation after a traditional lab test are virtually non-existent with these quick tests, often delivering competent results in under half an hour! This fast-paced result mechanism not only saves time but also significantly reduces anxiety. Indeed, this blend of convenience, speed, and immediate insight that quick tests using lateral flow technology provide, allows individuals and communities alike to make more timely and informed decisions regarding their health and well-being.
Importance of Testing in Controlling The Spread of The Virus
The critical role that testing plays in halting the spread of the virus is nearly impossible to overemphasize. At the very core, testing is our primary tool to identify those carrying the virus, enabling crucial protection measures to be taken. When an individual tests positive for the virus, they can then be appropriately isolated to prevent the virus from reaching more people.
Moreover, testing is instrumental in facilitating contact tracing. This is an essential process that involves identifying anyone who may have been in proximity to an infected person and consequently might be at risk. By informing these individuals of their possible exposure, they can take the necessary precautions, such as quarantining or getting tested themselves, thereby intercepting potential transmission chains.
On a larger scale, comprehensive testing lends invaluable data to governments and public health organizations. With a more accurate and extensive picture of the virus’s spread and impact severity, these authorities can more effectively tailor their responses. This could involve stricter lockdown measures in heavily impacted areas, ramping up vaccination drives, or even easing restrictions where the virus spread appears under control.
COVID-19 test kits prove to be a powerful shield in our ongoing war against this pandemic, working towards public health safety. However, the importance of these kits does not just stop at diagnosing the infected individuals. As we move forward in this blog post, we will delve deeper into how testing transcends merely revealing who is infected and instead plays several significant roles in our larger public health strategy to tackle this world problem.
COVID-19 Test Kits and Their Purpose
COVID-19 test kits are state-of-the-art diagnostic tools that have been developed expressly to identify the presence of SARS-CoV-2, the unique virus responsible for the global COVID-19 pandemic. These fascinating test kits are constructed to be highly sensitive and accurate in detecting the unmistakable signs of the virus, therefore making them indispensable tools in the fight against this disease.
Their fundamental objective is to pinpoint those individuals who have been infected by the virus. They do this by searching for the specific markers or signs of the virus in the individual’s body. This includes people who have symptoms commonly associated with COVID-19 – such as a high temperature, constant coughing, or a sudden loss of taste or smell – and those who do not have any symptoms but still carry the virus. These people, although asymptomatic, are just as capable of transmitting the virus to others.
Identifying infected individuals is a paramount step because it then enables further actions to be taken. This could encompass measures such as isolating the infected person at home or in a medical facility, providing the appropriate medical treatment to help ease symptoms and fight off the virus, or doing contact tracing to find out who else the person might have infected.
The crucial role these COVID-19 test kits play is clear: by facilitating the early identification of infected individuals, they allow for swift preventative measures to stop the virus from spreading further. Without such tests, we would be in the dark about who is infected and who isn’t, leading to an uncontrolled spread of the virus.
Furthermore, the data gathered from these test kits is an invaluable resource for national and global health organizations. It enables these entities to build a comprehensive understanding of the virus’s patterns and trends, as well as to develop effective containment strategies. The directions of lockdowns, travel restrictions, and vaccination initiatives are often determined based on the results of these tests.
How COVID-19 test kits work
COVID-19 test kits work by detecting the presence or absence of SARS-CoV-2. The PCR tests work by amplifying viral RNA present in a nasal or throat swab sample, followed by detection. Antigen tests detect the presence of specific viral proteins in the sample. Antibody tests, on the other hand, use a blood sample to identify the presence of specific antibodies that the body generates in response to the virus. Each of these methods plays an integral role in mapping the virus’s extent in a population and the body’s immune response against it.
The Role of COVID-19 Test Kits in Virus Containment
The role of COVID-19 test kits in controlling the ongoing spread of this stubborn virus cannot be overstated. These handy medical tools serve as the frontline of defense in our global battle against this unprecedented health crisis.
To start with, these kits provide the crucial first step toward understanding the scope of the infection. COVID-19 test kits work by diagnosing if an individual has the virus or not. If the person tests positive, immediate actions like quarantine and treatment can be taken. This simple act prevents the infected person from unknowingly spreading the virus to others. In this way, these test kits allow us to nip the spread of the virus in the bud.
Moreover, on a bigger picture, widespread testing gives health officials a broader understanding of how far the virus has spread in a community. It provides valuable data that contributes to tracing efforts, which further helps with isolating infected individuals and containment strategies. In many ways, it’s like putting a jigsaw puzzle together – each test kit provides one piece of the puzzle. On top of that, these COVID-19 test kits also proved to be essential in curbing outbreaks in high-risk locations such as nursing homes or hospitals where the most vulnerable populations reside.
Lastly, widespread testing using these kits could also give us the confidence to slowly reopen our society, knowing who’s infected and who’s not. It assists in making informed decisions about traveling and reopening schools, businesses, and other public places.
The crucial role of COVID-19 test kits in controlling the spread of this persistent virus highlights their importance. They help in patient isolation, drawing containment strategies, understanding the virus’s extent, and giving confidence in safely resuming normal life. As we navigate through this health crisis, these kits can be the guiding map that leads us to a safer destination. The more we leverage them, the more chance we have of winning this fight against COVID-19.
Detecting Asymptomatic Carriers and Preventing Further Transmission
One major challenge with COVID-19 is that some people show no symptoms but can still spread the virus. These asymptomatic carriers can unknowingly infect others. Luckily, effective COVID-19 test kits can identify these invisible spreaders, even in the absence of symptoms. Once identified, these individuals can be isolated, thus preventing further transmission. This measure is particularly important in crowded places like schools, offices, and public transportation.
Identifying Hotspots and Implementing Targeted Interventions
Widespread testing using COVID-19 test kits also helps in identifying virus hotspots—regions with a high number of infections. By having these insights, health officials can:
- Redirect resources to high-risk areas
- Implement stricter safety measures
- Carry out mass testing and vaccination drives
Efficient testing, therefore, enables targeted, effective interventions to be implemented instead of blanket measures that can disrupt normal life and economic activity.
Monitoring the Effectiveness of Preventive Measures
COVID-19 test kits do more than just diagnose; they measure the effectiveness of preventive measures, helping public health officials understand how well strategies like social distancing, mask-wearing, and vaccination are working. Increasing or decreasing case numbers can indicate if policies need to be adapted. Moreover, they can also detect new virus strains, providing vital information in the ongoing fight against COVID-19. These humble test kits serve as our frontline tools in containing the virus and maintaining public health. They are our eyes in the persistent fog of this pandemic, guiding our strategies and actions.
Ensuring Accessibility and Availability of COVID-19 Test Kits
The importance of COVID-19 test kits in virus containment cannot be overstressed. These invaluable tools are our first line of defense, providing critical data for the formulation of effective responses and interventions. However, the global nature of the pandemic has brought about unique challenges, especially in matters relating to the accessibility and availability of COVID-19 test kits.
Challenges in Scaling up Testing Capabilities
Scaling up testing capabilities on a global scale has been a major hurdle. The explosion of demand resulted in shortages of key components used in the testing kits, such as reagents and swabs. Transportation disruptions due to lockdown restrictions further complicated delivery and logistics. Moreover, the unprecedented situation required a quick ramp-up of production and distribution, testing the capabilities of even the most advanced economies.
Strategies for Improving the Availability of Test Kits
Despite the numerous challenges, various strategic measures have been implemented to improve the availability of COVID-19 test kits. Some of these measures are as follows
- Scaling production
In recent times, businesses have made substantial strides in escalating their production capabilities. They’ve adopted a more aggressive approach and significantly boosted their manufacturing capacities. This isn’t merely a minor uptick; it’s a drastic, sweeping elevation that’s transforming the landscape of production.
Shrewd business leaders have come to realize that to keep pace with soaring demand, traditional methods of production simply won’t suffice. So, they’ve rolled up their sleeves and taken innovative steps to bolster their output and meet the insatiable market needs effectively. This kind of monumental growth in manufacturing capacity fuels increased productivity, translates into higher profit margins, and fosters a healthier economic climate. What’s even more fascinating is that these companies are breaking the mold and transcending traditional business rivalries. Companies that were once fierce competitors are now sharing resources and collaborating. They’re putting aside their differences and working in tandem, using shared knowledge and resources to amplify their productivity.
This unprecedented level of teamwork among business rivals speaks volumes about the shift in attitudes. They recognize the shared benefits that come from pooling resources and knowledge. They are reinventing themselves and creating synergies that were once unthinkable. So, these companies are not only drastically expanding their manufacturing capacity but also building bridges with their competitors. This strategic move to team up with rivals reflects their sheer determination to keep up with consumer demand and carve out a successful future in a rapidly evolving marketplace.
- Diversifying suppliers
In response to the economic and logistical challenges faced in recent times, countries around the globe have been adopting a strategic approach to ensure stable and efficient supply chains. They have started diversifying their sourcing methods, aiming to reduce an over-reliance on a single supplier. This approach has been taken to minimize potential risks and maintain a significant level of self-sufficiency. This means that rather than depending solely on one nation or company for their essential goods or services, these countries are now broadening their horizons. They are establishing connections with a variety of suppliers worldwide. By doing so, they are not only enhancing their potential to secure goods and services but also enabling themselves to have options and backup plans to avoid potential shortages or delays. Undoubtedly, this trend of diversification reflects a key shift in global trade dynamics, emphasizing the importance of flexibility and adaptability in maintaining robust international supply chains.
- Rapid approval of new tests
Regulatory authorities have made huge strides in optimizing their approval process, paving the way for new and effective tests to be introduced to the market at a significantly faster rate. This acceleration in the approval process has been achieved by adopting streamlined procedures that simplify the regulatory journey for novel tests. By reducing bureaucratic red tape and expediting the review process, these regulatory bodies ensure that breakthrough tests are brought to the public swiftly, without compromising their safety and efficacy. In fact, with the streamlined approval process, we are witnessing an exciting era where promising and innovative tests can reach and benefit consumers in record times, enabling quicker, more effective detection and treatment of a range of conditions.
Ensuring Equitable Distribution of Test Kits
As we continue battling the virus, it is vital to ensure equitable distribution of test kits. Vulnerable populations, remote regions, and those with fewer resources are at particular risk of being overlooked. Governments and organizations are working closely to ensure that everyone has fair access to these lifesaving tools. Strategic geographical distribution, prioritization of high-risk areas, and implementation of mobile testing stations are some of the strategies being employed to achieve an equitable distribution of COVID-19 test kits.
The Impact of COVID-19 Test Kits on Public Health
Let’s explore this fascinating subject in more depth. One of the profound influences of COVID-19 test kits lies in the realm of public health. First, these test kits have been instrumental in quick detection. It’s no secret that the key to managing any infectious disease, especially on a global scale, begins with identifying who’s infected. Early detection made possible by these COVID-19 test kits allows for immediate isolation that prevents further spread of the virus.
Secondly, the data gathered by these tests has provided invaluable insights. Through this, we can better understand the virus – its spread, its severity – and create strategies to combat it. By analyzing the patterns and trends in the data, health authorities and researchers can make informed decisions, which could include implementing lockdowns or easing restrictions.
Thirdly, the COVID-19 test kits have been crucial in providing peace of mind to the public. People who suspect they might have been exposed to the virus can take a test and, depending on the result, either take appropriate medical action or breathe a sigh of relief. Knowing one’s status helps reduce uncertainty and anxiety amidst this global pandemic.
Lastly, the availability of these test kits has re-emphasized the importance of health infrastructure. It illustrates how equipped we must be in terms of medical supplies and facilities to handle such pandemics in the future.
The role of COVID-19 test kits in shaping public health cannot be understated. They help prevent the virus spread, provide vital health data, and offer peace of mind to individuals. They also highlight the need for strong and prepared health infrastructure. This depth of impact truly indicates the critical connection between COVID-19 test kits and public health.
Reducing the burden on healthcare facilities
COVID-19 test kits have considerably reduced the burden on healthcare facilities. Before the advent of these test kits, diagnosing the virus required extensive laboratory procedures, which took time and put immense pressure on healthcare professionals. However, the introduction of these kits has considerably streamlined this process. Now, diagnosing is not only quicker, but also reduces the need for hospital admission, especially for mild cases of the virus. This has eased the pressure on our already strained healthcare system.
- Quicker diagnosis
- Less time and resource-consuming
- Reduced need for hospital admission for mild cases
Enabling early detection and treatment
Another crucial role that COVID-19 test kits play is in early detection and subsequent treatment. The more efficient and widespread the testing, the quicker infected individuals can isolate and receive medical care. This early intervention proves crucial in halting the progression of the disease and improving patient outcomes.
- Efficient testing leads to early isolation
- Early medical intervention halts disease progression
- Improved patient outcomes
Supporting contact tracing efforts
These test kits are indispensable in supporting contact tracing efforts. Once an asymptomatic or mild case is confirmed, all recent contacts of the person can be identified. Those contacts can subsequently be tested, and if positive, isolated to prevent the further spread of the virus. In this way, COVID-19 test kits contribute significantly to virus containment.
- Easy identification of contacts with confirmed cases
- Allows for targeted testing and isolation measures
- Contributes significantly to virus containment
Challenges and Limitations of COVID-19 Test Kits
Despite the existential role they play, even COVID-19 test kits have their share of challenges and limitations that could potentially hamper the task of virus containment.
False negatives and False positives
Among the most significant issues are the risks of false negatives and false positives. The former means that a person has the virus but the test says they don’t — this could lead to further spread of the virus due to a false assurance of health. Meanwhile, false positives — where the test erroneously indicates that a person has the virus — can result in unnecessary quarantines and panic. Both scenarios underscore the importance of continuous refinement in testing technologies and the adoption of meticulous procedural controls.
Supply chain issues and quality assurance
The global demand for COVID-19 test kits is creating a big strain on supply chains, sometimes leading to shortages. With the pandemic continuing, the world is ordering more and more test kits, pushing the demand to an all-time high. This is creating pressure on supply chains more than ever before. If this continues, we might face long-term disruptions in the supply of these vital kits. Another tough part is ensuring these test kits meet quality standards. With everyone racing to produce more test kits quickly, we have to be careful. Speeding up production could risk the quality of the kits. Any production mistakes could result in testers that aren’t up to standard getting into the market. This could lead to incorrect test results, potentially causing the virus to spread further, false negative results, and a misinterpretation of the pandemic’s reach. We must not skip or rush quality checks, no matter how urgently these kits are needed. We have to make sure that producing more kits doesn’t compromise their accuracy and reliability. Manufacturers have a tough job: they need to keep up with the sky-high global demand while also making sure these life-saving test kits are high quality. In simple words, they need to produce more without letting the quality drop.
Addressing public skepticism and misconceptions
Public skepticism about the reliability of COVID-19 tests and misconceptions about the testing process itself poses significant challenges. Such assumptions could discourage people from getting tested, thus slowing down the efforts of virus containment. Public health education and transparent communication about the testing process are pivotal in overcoming these obstacles.
Innovations and Future Developments in COVID-19 Testing
Ever since the COVID-19 pandemic surge became global, testing has taken center stage in efforts to control the virus’ spread. Innovations and future developments in COVID-19 testing provide hope and momentum in our ongoing battle against this health crisis.
Advances in testing technologies
Advancements in testing technologies have played a significant role in enhancing our testing capabilities. We’ve seen the advent of saliva-based tests and pool testing methods, which can test multiple samples at once. These advances have facilitated quicker results, enabling more effective virus containment. Meanwhile, scientists are diligently working towards developing even more sophisticated testing technologies that can detect variants of the virus.
- Saliva-based tests are non-invasive and can be self-administered
- Pool testing methods provide quick results for large groups
- Ongoing research for detecting virus variants to stay ahead of the curve
Development of rapid and at-home test kits
The development of rapid test kits and at-home test kits marks huge strides in our fight against COVID-19. These kits empower individuals to carry out preliminary tests at their convenience, in a safe, controlled environment. They are critical in reducing stress on healthcare facilities and minimizing the risk of virus spread.
- Rapid test kits give results in minutes rather than hours or days
- At-home test kits allow for remote testing, limiting exposure risk
The role of testing in post-pandemic recovery
Testing will continue to play a crucial role in post-pandemic recovery. Regular testing can help monitor infection rates, detect new virus strains, and strategize effective containment measures to facilitate safe reopening. It remains one of the vital tools in our arsenal to ensure safety as we gradually return to normalcy.
- Regular testing can track infection rates
- Detects emerging variants of the virus
- Helps strategize containment measures for safe reopening.
Leading Manufacturers For COVID-19 Test Kits
Before we conclude, we want to introduce you to Medzell, a forward-thinking B2B platform designed to promote medical devices in emerging markets. Medzell facilitates connections between medical device manufacturers and healthcare providers, ensuring that cutting-edge medical equipment reaches the right hands across the globe.
AFFISPIN COVID-19 VIRAL RNA ISOLATION KIT
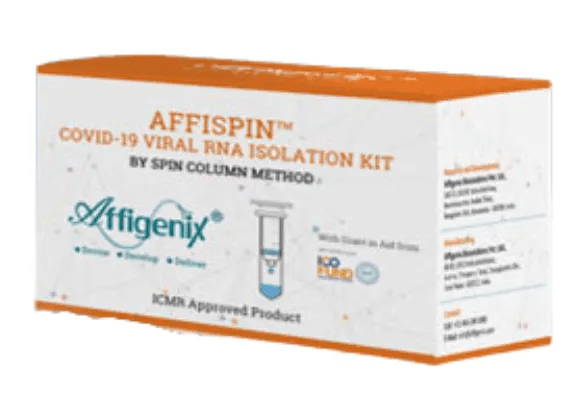 The AFFISPINTM COVID-19 VIRAL RNA ISOLATION KIT, a highly regarded product manufactured by Affigenix Biosolutions Pvt. Ltd., offers an effective and efficient method for isolating viral RNA. Affigenix Biosolutions, is globally recognized as a leading manufacturer and supplier of COVID-19 test kits. This specific kit provides 100% sensitive and accurate nucleic acid isolation, ensuring highly reliable results. It employs a simple, convenient, and rapid process, allowing RNA isolation in under 30 minutes. Available in two sizes for optimal convenience, there are options for either 50 or 100 reactions per kit. The product’s format may vary based on end-user requirements. A significant feature is the spin-column technique, which further enhances the kit’s effectiveness.
The AFFISPINTM COVID-19 VIRAL RNA ISOLATION KIT, a highly regarded product manufactured by Affigenix Biosolutions Pvt. Ltd., offers an effective and efficient method for isolating viral RNA. Affigenix Biosolutions, is globally recognized as a leading manufacturer and supplier of COVID-19 test kits. This specific kit provides 100% sensitive and accurate nucleic acid isolation, ensuring highly reliable results. It employs a simple, convenient, and rapid process, allowing RNA isolation in under 30 minutes. Available in two sizes for optimal convenience, there are options for either 50 or 100 reactions per kit. The product’s format may vary based on end-user requirements. A significant feature is the spin-column technique, which further enhances the kit’s effectiveness.
CoviConfirm Covid-19 Rapid Antigen Test
![]() CoviConfirm Covid-19 Rapid Antigen Test is a crucial diagnostic tool for detecting the presence of the COVID-19 antigen in individuals. Manufactured by Alpine Biomedicals Pvt. Ltd., this highly sensitive test boasts a sensitivity rate of 98% and provides accurate results with an impressive specificity rate of 100%. The convenience of specimen collection is made possible through nasal or nasopharyngeal swabs, requiring only a small sample volume. This testing kit ensures no cross-reaction to other microbes such as Influenza, Adenovirus, and TB, among others, supporting its specificity. With a long shelf life of 24 months when stored at 2-30°C, the kit proves itself a reliable addition to any healthcare setting.
CoviConfirm Covid-19 Rapid Antigen Test is a crucial diagnostic tool for detecting the presence of the COVID-19 antigen in individuals. Manufactured by Alpine Biomedicals Pvt. Ltd., this highly sensitive test boasts a sensitivity rate of 98% and provides accurate results with an impressive specificity rate of 100%. The convenience of specimen collection is made possible through nasal or nasopharyngeal swabs, requiring only a small sample volume. This testing kit ensures no cross-reaction to other microbes such as Influenza, Adenovirus, and TB, among others, supporting its specificity. With a long shelf life of 24 months when stored at 2-30°C, the kit proves itself a reliable addition to any healthcare setting.
Covid 19 Ag Microlisa
 J. Mitra & Co. Pvt. Ltd., one of the top manufacturers and global suppliers of medical test kits, presents the COVID-19 Ag Microlisa. Using human nasopharyngeal, nasal, and oropharyngeal swab samples collected in VTM or a sample diluent, this test kit enables in vitro qualitative detection of the COVID-19 nucleocapsid antigen. Based on the ELISA Sandwich concept and utilizing a conjugated enzyme for detection, the complete test can be conducted in a total of 120 minutes. The COVID-19 Ag Microlisa is a crucial screening tool for early SARS-CoV-2 infection detection.
J. Mitra & Co. Pvt. Ltd., one of the top manufacturers and global suppliers of medical test kits, presents the COVID-19 Ag Microlisa. Using human nasopharyngeal, nasal, and oropharyngeal swab samples collected in VTM or a sample diluent, this test kit enables in vitro qualitative detection of the COVID-19 nucleocapsid antigen. Based on the ELISA Sandwich concept and utilizing a conjugated enzyme for detection, the complete test can be conducted in a total of 120 minutes. The COVID-19 Ag Microlisa is a crucial screening tool for early SARS-CoV-2 infection detection.
Coviscreen, a COVID-19 testing kit by Tulip Diagnostics (P) Ltd, encompasses a comprehensive toolkit for SARS-CoV-2 antibody detection. In the Coviscreen kit, you’ll find individually pouched devices, sample droppers, and a ready-to-use buffer for testing. Offering a quick, in vitro diagnostic with a double antigen sandwich immunoassay, Coviscreen ensures the detection of Total antibodies (IgA+IgM+IgG) of SARS-CoV-2 virus in serum, plasma, and whole blood. This allows for faster detection and isolation of infected individuals. This testing kit is specially designed for mass testing, accommodating both patient-site and laboratory settings.
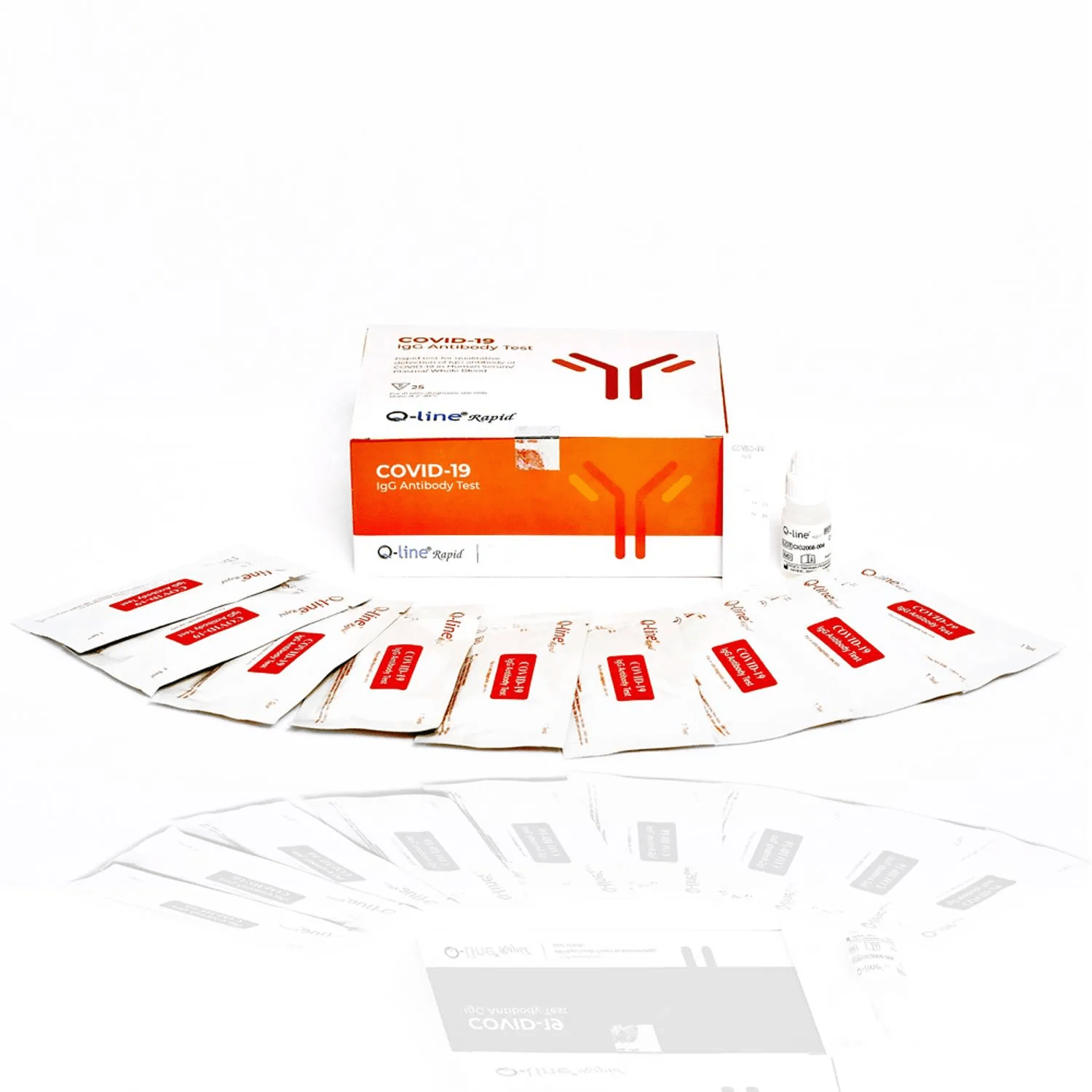
Q-line COVID-19 IgG Antibody Detection Kit
The Q-line Coronavirus (COVID-19) IgG Antibody Test Kit, a product of Q-line Biotech Private Limited, is an essential tool for detecting SARS-CoV-2 specific IgG antibodies in human plasma and serum. This user-friendly, cost-effective test is invaluable for Sero-surveillance and mass screening. Delivering results in just 15 minutes, this kit allows for efficient testing using whole blood, serum, or plasma samples. Persistence of the COVID-19 IgG antibodies in the human body, as detected by the test kit, provides a solid indication of patients who have been infected and are likely to have developed immunity to the virus.
Conclusion
Let’s picture a world where swift, cost-effective, and readily available COVID-19 tests are not an exception, but the standard way of life. Picture a world in which everyone, from the energetic, always-on-the-move city-dweller to the person living in the most isolated, far-flung corners of the globe, enjoys equal access to these vital testing tools that are indispensable for their safety and health. This isn’t just a fanciful dream, but a tangible possibility that holds immense promise. A world where COVID-19 tests are available for all puts us firmly on the route to overcoming this unprecedented health crisis, one that has left no stone unturned and has stretched its cruel grip to every nook and cranny of our diverse planet. Imagine not feeling helpless in the face of the pandemic but empowered, knowing that testing tools are available no matter where you are, who you are, or what your circumstances might be. Because in a world as interconnected as ours, no one is safe until everyone is safe.
But this vision can only come to life if we all do our part. It requires global solidarity like no other, a unified front against the virus, and a shared commitment to ensuring everyone has access to the defense weapons they need no matter their geographical or financial constraints. Let’s unite our voices and our efforts to bring this dream to fruition and turn the tide against COVID-19. A simple test could mean the difference between uncertainty and safety, risk and protection, fear and confidence. Together, we can make this vision our shared reality. Because it’s not just about overcoming the pandemic; it’s about forging a stronger, healthier, safer world for us all.